
Pressure in general is defined as force per unit area.
Gas pressure: Gas pressure in a container is the amount of force exerted per unit area by the moving gas molecules due to their collision with the surface wall. Pressure increases or decreases depending on the gas temperature, the number of molecules present, and the volume of the container.
|
Pressure Units |
|
pascal (Pa). 1 Pa = 1 N/m2 (recommended IUPAC unit) |
|
kilopascal (kPa). 1 kPa = 1000 Pa |
|
pounds per square inch (psi). air pressure at sea level is ~14.7 psi |
|
atmosphere (atm). 1 atm = 101,325 Pa = 760 torr air pressure at sea level is ~1 atm |
|
bar (bar, or b) 1 bar = 100,000 Pa (exactly) |
|
millibar (mbar, or mb). 1000 mbar = 1 bar |
|
inches of mercury (in. Hg). 1 in. Hg = 3386 Pa |
|
torr 1 torr = 1/760 atm |
|
millimeters of mercury (mm Hg). 1 mm Hg ~1 torr |
The relationships between the various macroscopic physical properties of gases - pressure, volume, number of molecules, and temperature - are established using the ideal gas law.
The ideal gas law is expressed using the following formula:
PV = nRT
Where :
P = pressure
V = volume
n = number of molecules
R = gas constant
T = temperature
This law demonstrates that the pressure of a gas is inversely proportional to the volume when other variables (such as the number of molecules and temperature) are kept constant. In other words, as the volume increases, the pressure decreases - and vice versa - as shown in the animation below (the green blocks in the animation represent pressure).
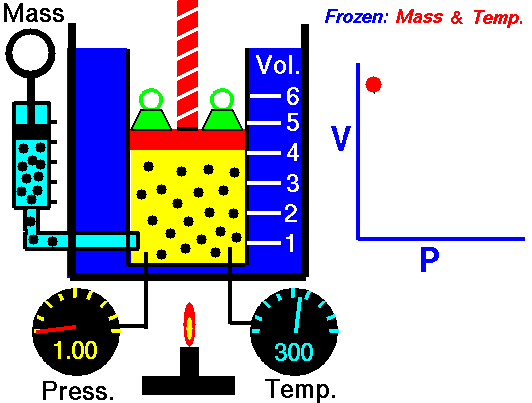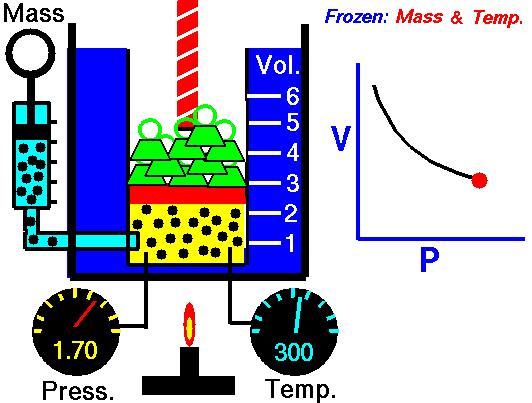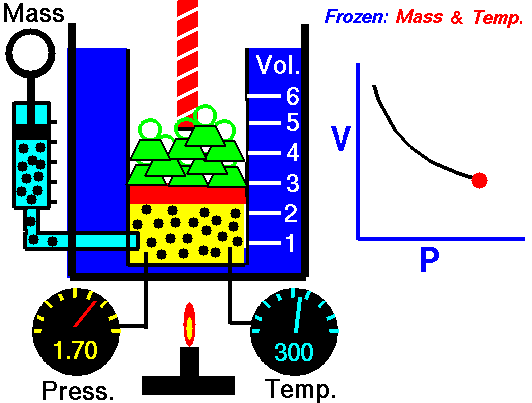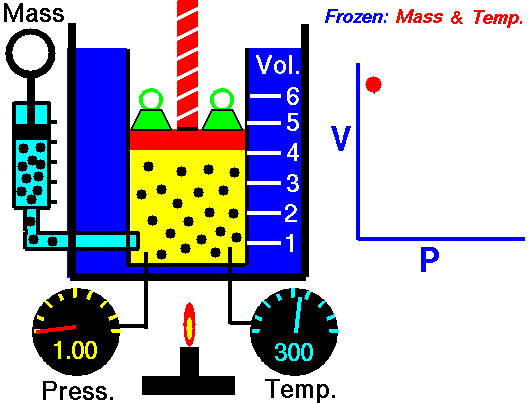
Figure 1: Animated Boyle's Law. From Tom Benson (2015, May 5) for the Glenn Research Center, NASA. https://www.grc.nasa.gov/WWW/K-12/airplane/aboyle.html
PiVi = PfVf
Where :
Pi = initial pressure
Vi = initial volume
Pf = final pressure
Vf = final volume
Charles' law demonstrates that when the pressure of a dry gas sample is kept constant, its volume is directly proportional to its temperature (in Kelvin) as shown in the animation below (the green blocks in the animation represent the pressure).
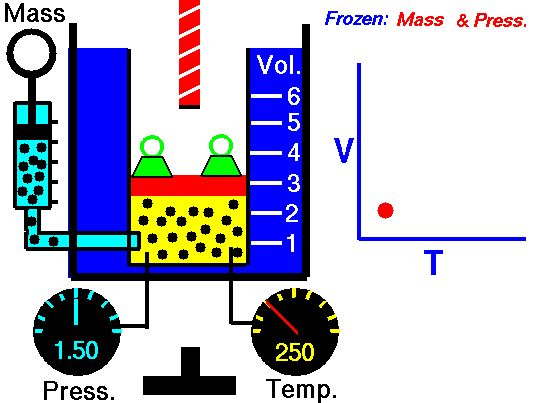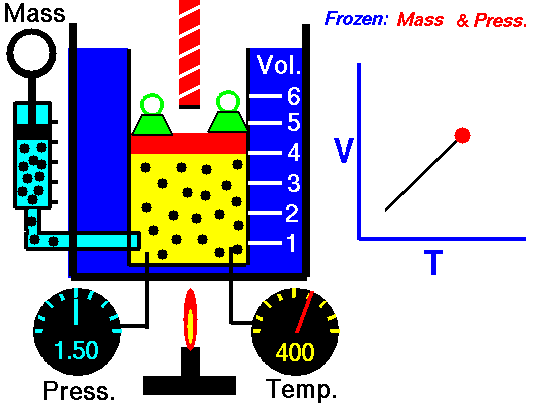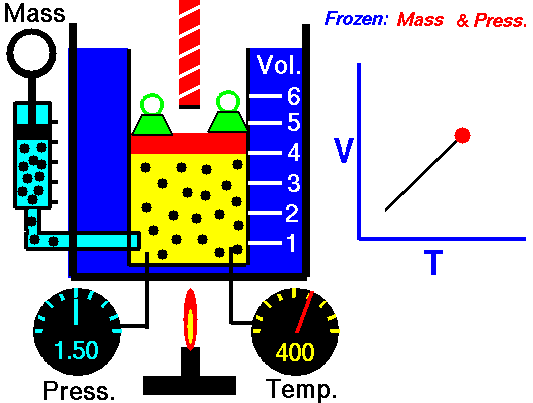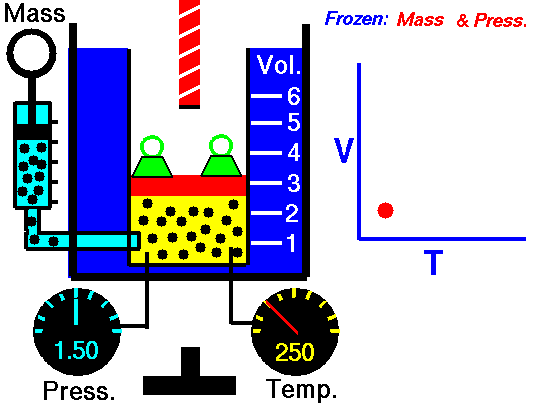
Figure 2: Animated Charles and Gay-Lussac's Law. From Tom Benson (2015, May 5) for the Glenn Research Center, NASA. https://www.grc.nasa.gov/www/k-12/airplane/aglussac.html
Where :
Ti = initial temperature
Vi = initial volume
Tf = final temperature
Vf = final volume
Boyle's law and Charles' law are combined into form combined gas law as shown below.
Some of the content of this guide was modeled after a guide originally created by Openstax and has been adapted for the GPRC Learning Commons in November 2020. This work is licensed under a Creative Commons BY NC SA 4.0 International License.